BACKGROUND
Allogeneic hematopoietic cell transplant (alloHCT) may be a curative treatment option for patients with myelodysplastic syndrome (MDS). The use of investigational therapies that potentially reduce transplant toxicity could improve outcomes in this patient population with difficult to treat disease. Per CIBMTR, survival after alloHCT for MDS using matched donors is approximately 65% at 1 year and relapse-free survival at 1 year was ~69% in MDS patients in the RICMAC trial who received myeloablative conditioning (MAC).
Orca-T is a high-precision cell therapy biologic that includes stem and immune cells, derived from allogeneic donors, that leverages highly purified, polyclonal donor regulatory T cells to control alloreactive immune responses. In this phase 1b study, Orca-T was evaluated in MDS patients who were eligible for transplant per 2017 International Expert Panel recommendations.
METHODS
As of 03/31/23, 16 patients with intermediate to very high risk (HR) MDS with ≤10% blasts in the bone marrow at the time of transplant had received Orca-T in the phase 1b study. IPSS-M prognostic scores were Low (6%), Moderate Low (6%), Moderate High (19%), High (44%), and Very High (25%). Baseline HCT-CI scores were 0 (n=6), 1 (n=3), 2 (n=2), 3 (n=3), and 4 (n=2). The median f/u was 19.1 months (range 6.3 - 32.2). Patients were aged 43 - 65 (median 59) and 56% female. Donors were 8/8 HLA-matched related (n=7) or unrelated (n=9). Patients received a busulfan-based myeloablative chemotherapy regimen prior to Orca-T, followed by single-agent GvHD prophylaxis with tacrolimus.
RESULTS
Orca-T was successfully manufactured in a single, centralized GMP manufacturing facility for all patients enrolled, distributed, and infused throughout the U.S. Orca-T was delivered within < 72 hours vein-to-vein (i.e. time between end of donor apheresis to start of recipient's Orca-T infusion).
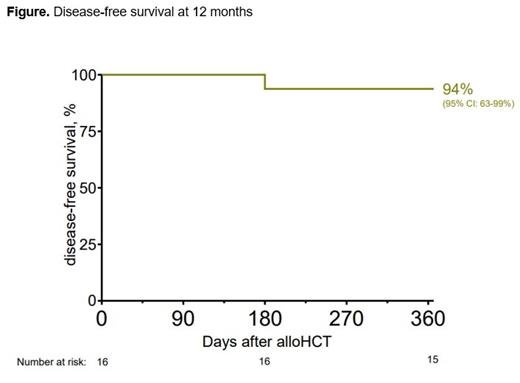
Disease-free survival (RFS) at 12 months was 94% (Figure). At 1 year, no patients had experienced grade ≥ 3 acute GvHD and moderate to severe chronic GvHD had occurred in 2 patients. Modified GRFS (survival free of Grade 3-4 aGvHD, moderate-to-severe cGvHD and relapse) at 12 months was 75%. Non-relapse mortality (NRM) and overall survival (OS) were 0% and 94%, respectively, at 12 months.
Additionally, one MDS patient with very poor prognosis, characterized by mutated TP53 and complex karyotype, remains in CR without active signs of acute or cGvHD at 500 days post-alloHCT.
CONCLUSIONS
Orca-T demonstrated promising results in this HR-MDS patient population, including high RFS, low incidence of GvHD, and very high OS at 1 year. A randomized controlled phase 3 multi-center trial comparing Orca-T to SOC in patients with MDS, AML, and ALL is currently enrolling across the US (NCT05316701).
Disclosures
Gandhi:orca bio: Research Funding. Hoeg:Orca Bio: Research Funding. Patel:Kite Pharma: Speakers Bureau; CTi BioPharma: Consultancy; Sanofi: Speakers Bureau; orca bio: Research Funding. Waller:Cambium Medical Technologies: Current equity holder in private company, Other: Founder; PartnersTherapeutics: Research Funding; Secura: Research Funding; NCI R01: Research Funding; Sanofi: Research Funding; BMS: Research Funding; ORCA: Research Funding; CSL Behring: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; CRISPR: Consultancy; Allovir: Consultancy; Verastem: Consultancy, Research Funding; Cambium Oncology: Current equity holder in private company, Other: Founder. Pantin:Orca Bio: Research Funding; Omeros, Sanofi: Speakers Bureau; Omeros: Honoraria. Oliai:Orca Bio: Research Funding; Novartis: Research Funding; Seagen: Research Funding; Arog: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding. McClellan:Orca Bio: Current Employment. Pavlova:Orca Bio: Current Employment. Agodoa:Orca Bio: Current Employment. Fernhoff:Orca Bio: Current Employment. Meyer:Orca Bio: Research Funding.